Simulation-based method to target Epilepsy goes into clinical trial
14 November 2019
In what represents a major milestone on the path to clinical application, a novel method to improve outcomes of Epilepsy surgery has now received approval for clinical testing in 13 French hospitals.
• Human Brain Project research provides basis for a possible personalized medicine advance in Epilepsy care
• New approach could bring first breakthrough in decades for drug-resistant patients undergoing brain surgery
08 11 2019 – In what represents a major milestone on the path to clinical application, a novel method to improve outcomes of Epilepsy surgery has now received approval for clinical testing in 13 French hospitals. The approach could provide a better therapeutic perspective against the drug-resistant form of the disease, which constitutes one third of all cases, and is a development by Human Brain Project scientist Viktor Jirsa and an interdisciplinary team of collaborators. To help clinicians plan surgery strategies, the scientists create personalized brain models of patients and simulate the spread of abnormal activity during epileptic seizures. The method represents the first example of a personalized brain modeling approach entering the clinic and will now be assessed over four years in a cohort of 356 patients under strict requirements.
Epilepsy is a wide-spread neurological disorder that affects around 50 million people worldwide. In many cases, the seizures that mark the disease can be controlled by drugs, but close to a third of all patients are drug resistant. For them, the only remaining option is surgical removal of the epileptogenic zone, the area from which the seizure activity first emerges and then spreads. During surgery preparation it is critical to localize this area as precisely as possible in the brain, but very challenging with current methods. As a result, surgery outcomes are difficult to predict, with success rates of only around 60%.
“This low success rate has largely stayed the same for the last 30 years. We hope our approach can finally improve the odds for patients”, says Prof. Viktor Jirsa. The scientist is Director of Inserm’s Institut de Neurosciences des Systèmes (INS) at Aix-Marseille University and Director of Research at CNRS. In the Human Brain Project he is the Deputy Leader of the research area of Theoretical Neuroscience.
Over the last five years and in large part within the framework of the Human Brain Project, Jirsa and his team worked on an approach that could bring a change. The team has adapted the open network simulator The Virtual Brain towards applications in Epilepsy. This work has laid foundations for the project EPINOV, short for “Improving EPilepsy surgery management and progNOsis using Virtual brain technology”, a consortium coordinated by Prof. Fabrice Bartolomei (Hôpital de la Timone) that brings together theorists like Jirsa, clinical neuroscientists, in particular from Marseille and Lyon, and the industry partner Dassault Systèmes.
After two pilot studies showed promising results for the approach, the EPINOV-consortium has received approval from the French regulatory authority to put their approach to the test in a full-scale multi-centric trial with almost 400 prospective patients.
“It represents the world’s first clinical trial ongoing using full brain network modeling,” says Jirsa. “When the authorization came in, it was like a huge pressure was relieved from me after all this hard work. Then followed last preparations to assure that all steps in the workflow of virtualization and evaluation of the patient brains are in order during the four year period of the trial.”
Fabrice Bartolomei explains "This type of epilepsy affects millions of patients worldwide. The personalized modeling of epilepsy networks in drug-resistant patients is an innovative and scientifically validated approach, which proposes to enrich the interpretation of neurophysiological and neuroimaging tests, and thus to improve the surgical prognosis of epilepsy in an individualized way".
Viktor Jirsa and his close collaborators Profs. Randy McIntosh at Baycrest Center Toronto and Petra Ritter at Charité Berlin started building The Virtual Brain as an open source brain network simulation engine from 2010 on, using neuronal population models and structural information from neuroimaging.
“In the Human Brain Project environment the conditions were perfect to go the decisive steps further towards applying it in a clinical context. The science underlying this trial is almost entirely a result of our work in the HBP”, the scientist says.
First a personalized brain model is created from data on the individually measured anatomy, structural connectivity and brain dynamics for each patient. Through a series of steps it is turned into a dynamic model, on which the seizure propagation can be simulated. High Performance Computing enables the personalisation of the brain network models through the application of machine learning. The resulting “brain avatar” is customized to the individual patient and allows testing and estimating during surgery preparation. “In a small cohort of retrospective surgery patients we were able to demonstrate that the predictions of the patient’s brain model correlate well with positive surgery outcome”, Jirsa explains, “and other labs have confirmed our results independently.” A detailed account on this work can be read here.
In half of the cases, the surgeons will have information from the Epilepsy-model in their staff meetings, where therapeutic interventions are planned. “It´s a blind random design, half of these patients will be operated taking our model predictions into account, the other half will not. After four years, the statistics will show us hopefully to what degree the model predictions changed the surgery practice, results, and outcome”, Jirsa says.
Within the Epinov consortium the industrial partner Dassault Systèmes will develop a virtual brain-based simulation software prototype that could subsequently be provided to clinics worldwide. Headquartered in France, Dassault Systèmes is a multinational software company focused on 11 industries including life sciences and the development of patient-centric modeling and simulation experiences.
“There is a very big responsibility”, Jirsa emphasizes “and at the same time it’s very exciting that we have this chance to improve clinical practice and ultimately patients’ lives. And if the approach succeeds, it would also be the first modelling-based example of personalized medicine that makes the jump from research to clinical practice – so the outcome will certainly be a signal to the field”.
Prof. Katrin Amunts, Scientific Research Director of the Human Brain Project, highlights the significance of the move into the clinic: “This breakthrough by Viktor Jirsa and his colleagues is a fantastic example of a new type of technology-enabled computational neuro-medicine. It is one of our central aims to catalyze developments like this that make concrete contributions in the fight against brain diseases to benefit patients.”
Prof. Philippe Ryvlin, who leads the Medical Informatics Platform in HBP and the epilepsy surgery section of the European Reference Network EpiCARE emphasises that “this clinical trial, which will be the largest randomised study ever performed in epilepsy surgery, demonstrates that simulation of the human brain, as developed in HBP, has now reached a stage where it can be readily applied to address unmet medical needs.”
Virtual Brain Researchers are also continuing their activities on clinical modeling for stroke and Alzheimer’s in collaboration with experts that the HBP brings together. For Jirsa seeing his work as a theoretical scientist gain this potential impact has been the result of a unique convergence: “That we have come to this point was made possible by clinical expertise and our activities on The Virtual Brain and the Human Brain Project all coming together – right place, right time, right people."

Viktor Jirsa
Viktor Jirsa is Director of the Inserm Institut de Neurosciences des Systèmes at Aix-Marseille-Université. Dr. Jirsa is a theoretical neuroscientist working in the field of brain connectivity and brain network modeling. He leads the multi-scale brain connectome efforts in the Human Brain Project and is Curator of the neuroinformatics platform The Virtual Brain (http:www.thevirtualbrain.org). (Photo: private)
Contact
Prof. Viktor Jirsa
Email: viktor.jirsa@univ-amu.fr
tel : ++33 (0)4 91 32 42 51
Media Contact:
Peter Zekert
Human Brain Project
Public Relations Officer
Tel.: +49 (0) 2461 61-96860
Email: press@humanbrainproject.eu
Further Material:
Photos (© INS UMR 1106, high-res material available on request):
 The theorist and the clinician: Viktor Jirsa (left) has developed the modelling underlying the clinical trial. Neurologist Fabrice Bartolomei (right) is the clinical coordinator.
The theorist and the clinician: Viktor Jirsa (left) has developed the modelling underlying the clinical trial. Neurologist Fabrice Bartolomei (right) is the clinical coordinator.
Figures:
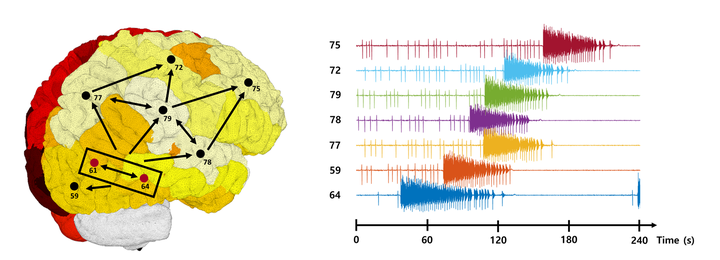
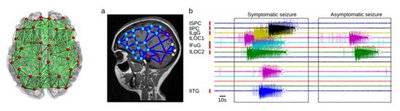
Figure 1: Based on brain network simulations using TVB, patient-specific seizure propagation characteristics can be reproduced. The figure shows a simulation result for a particular patient, in which seizure activity is generated from epileptogenic zones (nodes 61 and 64), and sequential recruitment is induced through the individual brain connectome. ©Ahn et al (2019)
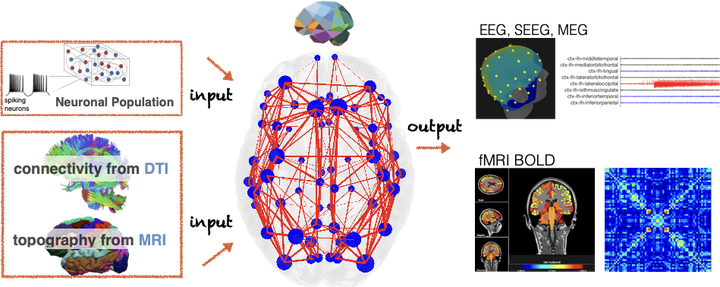 Figure 2: Combining non-invasive structural brain imaging data (anatomy and connectivity data from Magnetic Resonance Imaging (MRI) and Diffusion Tensor weighted Imaging (DTI)) with computational models of neuronal populations, patient-specific brain models can be created and used as personalized in-silico platforms for clinical hypothesis testing. The virtual brain models are capable of simulating human brain imaging routinely measured in hospitals, inlcuding electroencephalography (EEG), magnetoencephalography (MEG), stereotactic electroencephalography (SEEG), and functional Magnetic Resonance Imaging (fMRI measuring the blood oxygenation level dependent (BOLD) signal)). © Petkoski et al (2019)
Figure 2: Combining non-invasive structural brain imaging data (anatomy and connectivity data from Magnetic Resonance Imaging (MRI) and Diffusion Tensor weighted Imaging (DTI)) with computational models of neuronal populations, patient-specific brain models can be created and used as personalized in-silico platforms for clinical hypothesis testing. The virtual brain models are capable of simulating human brain imaging routinely measured in hospitals, inlcuding electroencephalography (EEG), magnetoencephalography (MEG), stereotactic electroencephalography (SEEG), and functional Magnetic Resonance Imaging (fMRI measuring the blood oxygenation level dependent (BOLD) signal)). © Petkoski et al (2019)
Original Publications:
Olmi S, Petkoski S, Guye M, Bartolomei F, Jirsa V: Controlling seizure propagation in large-scale brain networks. PLOS Computational Biology, Vol. 15, No. 2 2019-02-25. https://doi.org/10.1371/journal.pcbi.1006805
Proix T, Jirsa VK, Bartolomei F, Guye M, Truccolo W.: Predicting the spatiotemporal diversity of seizure propagation and termination in human focal epilepsy. Nat Commun. 2018 Mar 14;9(1):1088. doi: 10.1038/s41467-018-02973-y.
Proix T, Bartolomei F, Guye M, Jirsa VK (2017) Individual brain structure and modeling predict seizure propagation. Brain 140 (3): 641-654 https://www.ncbi.nlm.nih.gov/pubmed/28364550
Jirsa VK, Proix T, Perdikis D, Woodman MM, Wang H, Gonzalez-Martinez J, Bernard C, Bénar C, Chauvel P, Bartolomei F (2016) The Virtual Epileptic Patient: Individualized whole-brain models of epilepsy spread. Neuroimage doi :10.1016/j.neuroimage.2016.04.049
Further Information:
EPINOV
Improving EPilepsy surgery management and progNOsis using Virtual brain technology
http://www.epinov.com/
 This work is supported by a public grant overseen by the French National Research Agency (ANR) as part of the second "Investissements d'Avenir" program (reference: ANR-17-RHUS-0004).
This work is supported by a public grant overseen by the French National Research Agency (ANR) as part of the second "Investissements d'Avenir" program (reference: ANR-17-RHUS-0004).

Related News
JULY 1, 2019
The Human Brain Project – synergy between neuroscience, computing, informatics and brain inspired technologies
In a new PLOS Biology community page, leading scientists from the Human Brain Project give an overview of the project’s science and infrastructure approach, as well as opportunities for the scientific community to make use of novel computational ressources for neuroscience. https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000344
JUNE 3, 2019
The scientific case for brain simulations
HBP scientists argue for brain simulators as “mathematical observatories” for neuroscience
 Simulations of large-scale networks of neurons are a key element in the European Human Brain Project (HBP). In a new perspective article scientists from the HBP argue why such simulations are indispensable for bridging the scales between the neuron and system levels in the brain. The authors describe the need for open general-purpose simulation engines that can run a multitude of different candidate models of the brain at different levels of biological detail. Comparing predictions derived from such simulations with experimental data will allow systematic testing and refinement of models in a loop between computational and experimental neuroscience. The article has been published as a featured perspective in the leading journal Neuron.
Simulations of large-scale networks of neurons are a key element in the European Human Brain Project (HBP). In a new perspective article scientists from the HBP argue why such simulations are indispensable for bridging the scales between the neuron and system levels in the brain. The authors describe the need for open general-purpose simulation engines that can run a multitude of different candidate models of the brain at different levels of biological detail. Comparing predictions derived from such simulations with experimental data will allow systematic testing and refinement of models in a loop between computational and experimental neuroscience. The article has been published as a featured perspective in the leading journal Neuron.
https://www.humanbrainproject.eu/en/follow-hbp/news/the-scientific-case-for-brain-simulations/
NOV. 29, 2017
Epilepsy: Building Personalised Models of the Brain
 Human Brain Project scientist Viktor Jirsa is the head of a team creating personalised brain models for patients with intractable epilepsy. He explains the process and how the HBP’s brain models and cross-displinary collaborations are central to a new way of targeting epilepsy surgery.
Human Brain Project scientist Viktor Jirsa is the head of a team creating personalised brain models for patients with intractable epilepsy. He explains the process and how the HBP’s brain models and cross-displinary collaborations are central to a new way of targeting epilepsy surgery.
https://www.humanbrainproject.eu/en/follow-hbp/news/the-power-of-a-personalised-model-of-the-patients-brain/
Simulation engines in HBP
“The simulation aspect in HBP gives us the opportunity to bring together our macroscale-approach to modelling and simulation with a range of different simulation-based approaches. Scientifically, that lets us better understand how phenomena on the micro-level might translate to higher order phenomena on the meso- and macro-scale, and can open possibilities of a “consistency test” between the models.” Viktor Jirsa
NEURON and Arbor can run biophysically detailed, multicompartmental neuron models to help understand how dendritic structures affect the integration of synaptic inputs and, consequently, the network dynamics.
More abstracted simulators like NEST can simulate spiking networks of billions of simplified point neurons which model the basic integration of signals and firing of the neurons. This is close to the level of description widely used in AI today, allowing for connections of this type of brain simulations to technology development in this area as well as in robotics. In contrast to most current AI algorithms, NEST and HBP’s neuromorphic hardware systems already capture the fact that real neurons communicate using sparse but robust pulses, and thus operate in a very energy efficient way.
Whole brain-level simulation with the engine The Virtual Brain uses population firing-rate models that summarize dynamics of larger populations of neurons. Such coarse-grained simulation can already help understand and predict large-scale dynamics. Since alterations of such dynamics can be a feature of brain pathologies, like the seizure propagation in Epilepsy, this approach has led to first clinical developments based on patient-specific brain modeling.
About the Human Brain Project
Understanding the organisation of the human brain at all relevant levels is a big challenge, but necessary to improve treatment of brain disorders, create new computing technologies and provide insight into our humanity. Modern ICT brings this within reach. The HBP’s unique strategy uses it to gather, integrate and analyse brain data, understand the healthy and diseased brain, and emulate its computational capabilities. By sharing our tools with researchers worldwide, we aim to catalyse global collaboration.
Unlocking the brain’s secrets promises major scientific, social and economic benefits. One is improved diagnosis and treatment of brain-related diseases; a growing health burden in our ageing population. A second is neuroscience’s potential to contribute to approaches for future ICT, including extreme-scale and neuromorphic computing. The HBP will also contribute to a brain-inspired approach to Artificial Intelligence and robotics.
The HBP studies the brain at different levels, from genomics to higher-level brain functions. To help achieve this goal, the HBP is building an ICT-based research infrastructure to facilitate research collaboration, via the sharing of software tools, data and models. Incorporating inputs from the scientific community, the HBP’s scientists and engineers ensure that our infrastructure meets real research needs. Another aim is to accelerate medical research, by facilitating researchers’ secure access to broader data sets of patient data, as well as HBP tools and models. The HBP also educates young scientists to work across disciplinary boundaries and addresses the ethical implications of its work. Finally, it helps to integrate global brain research efforts and leads Europe’s contribution in this field.