HBP lead scientist Roshan Cools aims to make dopamine drug prescriptions more personal and effective
01 June 2022
Dopamine is involved in several brain disorders and healthy brain functions, and dopaminergic drugs are commonly used as treatments or to improve cognition. But the benefits of these drugs are not the same for everyone, as their effects vary both across individuals and within the same patient. Roshan Cools, a lead scientist at the Human Brain Project, is working to build a prediction model of dopamine drug effects on human cognition, combining pharmacological neuroimaging, receptor mapping and machine learning.
This could pave the way for personalised prescriptions for depression, ADHD, Parkinson's disease and others, allowing doctors to prescribe the right drugs to the right person at the right time.
Cools is Professor of Cognitive Neuropsychiatry at the department of psychiatry of the Radboud University Nijmegen Medical Centre and principal investigator at the Donders Institute for Brain, Cognition and Behaviour (Centre for Cognitive Neuroimaging). She recently received an Advanced Grant of the European Research Council (ERC) to study the cortical and cognitive control of substances in the brain that regulate emotions, thoughts and behaviours, including dopamine and serotonin.
We spoke with her about the goals and the challenges of this research.
As a lead scientist at the HBP, you are working to build a prediction model of dopamine drug effects on human cognition. What are the main goals of this research?
We've been interested in understanding who benefits in which context from therapeutic drugs that are quite commonly used in psychiatry and neurology, in particular dopaminergic drugs. Those drugs are used across different diagnoses: in Parkinson’s disease, schizophrenia, ADHD, and also by healthy people, for cognitive enhancement. But there is a lot of variability both across different individuals and also within the same individuals under different conditions.
The ultimate aim of our work is to predict who will benefit from these common drugs and in which context. That's quite an ambitious aim. We had good reason to believe that the effects of these drugs on cognition can be predicted from baseline levels of dopamine.
We've known for many years that there is a nonlinear relationship between dopamine receptor stimulation and cognitive performance: We perform best with intermediate levels of receptor stimulation. We perform poorly when there’s too little dopamine, but also when there’s too much.
To study the predictive value of baseline dopamine levels for dopaminergic drug effects, we recently acquired pharmacological brain imaging data from a relatively large sample, of 100 subjects, funded by an NWO Vici grant. We measured the effects of the drugs on cognitive performance, and, in the same subjects, also how much dopamine they already have in their system at baseline. The aim was to test whether we can predict on an individual basis if someone with low levels of dopamine benefits from a drug and someone with optimised levels actually gets worse, or performs more poorly after intake of the same drug dose.
How do the HBP resources facilitate this research?
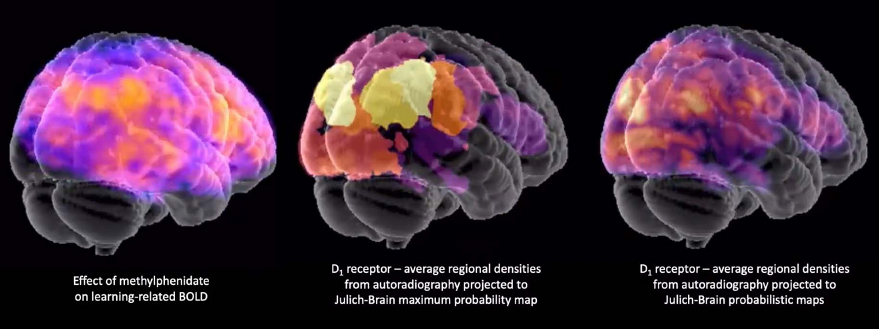
We wanted to use fMRI and PET imaging data to predict dopaminergic drug effects. That's very big data in the sense that you get signals from all over the brain. The development of the proxy prediction model would benefit greatly from constraining the predictor variables by prior knowledge about where the receptors are located, and in the HBP’s Julich Brain Atlas on EBRAINS there’s all these wonderful autoradiography data available and precise knowledge of the microstructure. We hope to leverage the microstructural information of the HBP’s Human Brain Atlas to constrain the imaging data in order to predict dopaminergic drug effects. By doing this, we can disentangle which effects are mediated by dopamine versus other receptors, crucial for optimally building the model.
More concretely, we want to select specific spatial regions in the brain based on where we see the most dopamine receptors, because those are the most likely to contribute to dopaminergic drug effects.
When this model is achieved, in which way could it help the clinical research community? What problems would it solve?
If we succeed – and that's quite a challenge, I have to admit – the clinical gain is knowing who will benefit from a dopaminergic drug. Then, we can prescribe the drug to the right person at the right time. That's the idea, to personalise and contextualise drug prescription.
This could solve problems related to the pharmacological treatment of many disorders that implicate dopamine, including depression. When clinical researchers look at the effects of antidepressants across samples of patients and see that only some people benefit from such drugs they might conclude that these drugs don’t work, full stop. However, there is enough evidence, also from work with experimental animals, to suggest that this is very unlikely. What’s much more likely is that they do work well, but only in some people: The challenge is to resolve the huge individual variability, identifying who will benefit from antidepressant drugs that act on the dopamine system and who instead would benefit from the targeting of a different one, for example, the serotonin system.
Instead of going down the list of treatments one by one, it would be an advantage if we had biomarkers to know which drug to prescribe to which person in which context. This holds for people with depression, with schizophrenia and with Parkinson’s disease
If we can build a model that includes PET scans, but also less invasive behavioural predictors, this will be beneficial for the clinical research community. And perhaps, more immediately, for the psychology community. There are many researchers interested in the mechanisms by which dopamine changes cognition and brain function. But we can only isolate these effects if we take into account this large individual variability.
What is currently known about the roles of dopamine in the brain?
On the one hand, dopamine seems to promote reward-driven impulsivity and compulsivity associated with drug addiction, and other impulse control disorders. On the other hand, dopamine is also well established as a cognitive enhancer. In ADHD, its action is increased by drugs like Ritalin (methylphenidate) and that actually helps people to be less distracted and less impulsive.
These paradoxical effects might reflect the observation that dopamine has very different effects depending on where it acts in the brain: While dopamine in the prefrontal cortex helps us focus and resist distraction, it can make us more impulsive by acting on the striatum where it boosts reward-associated habitual behaviours. We think these dual roles of dopamine are not just antagonistic, they do not just compete, but they can also be synergistic, working together to increase ‘striatal’ motivation for ‘prefrontal’ impulse control.
Dopamine receptor densities mapped onto the Julich Brain Atlas on EBRAINS
In what stage is your research right now, and what has proven surprising or challenging so far?
We've been able to show that the great variability across individuals in dopaminergic drug effects can indeed be accounted for by variation in baseline levels of dopamine synthesis capacity, measured with an invasive PET imaging technique.
We were also interested to see whether we can predict these effects not only from invasive PET measures, but also from more easily available behavioural proxies of dopamine. There have been quite a few indications that relatively simple, off-the-shelf measures of eye blink rate, impulsive personality and working memory capacity can be used to predict drug effects on cognition. So far, in our large sample of participants, we don’t see a very strong link between these simple fast-to-obtain measures and our PET measure of dopamine synthesis capacity or our drug effects. While this suggests that the simplest off-the-shelf behavioural measures cannot replace expensive and invasive direct PET measures of brain dopamine, we are now investigating whether measures of neural activity, obtained with less invasive functional magnetic resonance imaging (fMRI), can do a better job, particularly if we take into account knowledge about where in the brain the dopamine receptors are most abundant.
The challenge for building a proxy model is to select the right measures, to have a sufficient sample size, and to adopt the right statistical and analysis methods for validation, and we're in the middle of optimising all these factors.
The interview was conducted by Helen Mendes.